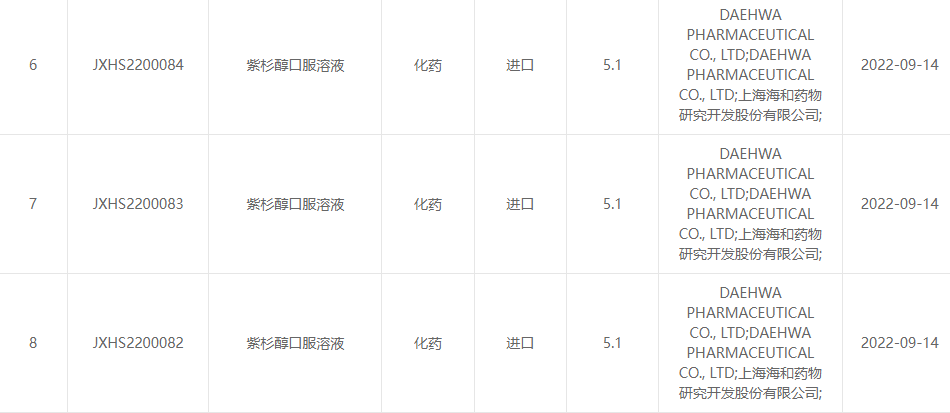
Na Septemba 13, 2022, Shanghai Haihe Pharmaceutical Research and Development Co., Ltd. na Daehwa Pharmaceutical Co., Ltd. jikọtara ọnụ kwuwapụta na paclitaxel oral solution (RMX3001) nke ndị otu abụọ mebere bụ nke Center for Drug kwadoro nke ọma. Nyocha (CDE) nke nchịkwa ọgwụ na steeti.( Nọmba nnabata: obodo JXHS2200082, obodo JXHS2200083, obodo JXHS2200084).
Isi iyi onyonyo: Nchịkwa ọgwụ steeti
Paclitaxela na-ejikarị agwọ ọrịa etuto dị iche iche dị ka ọrịa kansa akpa ume, ọrịa ara ara, ọrịa ovarian cancer, isi na olu, na ọrịa kansa afọ.Protein polymerization, mgbakọ microtubule, igbochi depolymerization, si otú ahụ na-eme ka microtubules guzosie ike ma na-egbochi mitosis nke mkpụrụ ndụ cancer na ịkpalite apoptosis, si otú ahụ na-egbochi mgbasa nke mkpụrụ ndụ cancer ma na-egwu ọgwụ mgbochi ọrịa cancer.
Ka ọ dị ugbu a, ihe ka ọtụtụ n'akụkụ ụwa na-eji paclitaxel n'ụdị injection, bụ nke a ga-emepụta ma na-enye ya site na ntapu intravenous n'ụlọ ọgwụ.Ndị ọrịa kwesịrị ịlaghachi n'ụlọ ọgwụ ugboro ugboro, a ga-enwekwa mmeghachi omume ọjọọ na saịtị ịgba ọgwụ.Ya mere, mmepe nke nkwadebe paclitaxel ọnụ na-abụkarị ebe dị ọkụ na nyocha ụlọ ọrụ..
RMX3001 bụ usoro ọnụ nke paclitaxel nke Dahua Pharmaceutical mepụtara na-adabere na nkà na ụzụ nnyefe ọgwụ na-emepụta egbugbere ọnụ ya.Ndị nchịkwa nri na ọgwụ ndị Korea kwadoro ya na Septemba 2016 (aha ahia Liporaxel), na ihe ngosi ahụ dị elu ma ọ bụ ọgwụgwọ nke abụọ nke ọrịa cancer gastric metastatic ma ọ bụ ọrịa cancer gastric na-emegharị ugboro ugboro.Dị ka akwụkwọ akụkọ sitere na Haihe Pharmaceuticals si kwuo, Liporaxel bụ ngwaahịa paclitaxel mbụ nke emepụtara nke ọma ma kwadoro maka ịzụ ahịa n'ụwa ruo ugbu a.Na Septemba 2017, Haihe Pharmaceutical nwetara R&D, ikike imepụta na ịre ahịa na China China, Hong Kong, Taiwan na Thailand sitere na Dahua Pharmaceuticals.
Ngwa ndepụta nke RMX3001 na China na-adabere na usoro a na-ahaziri ahazi, nke na-emeghe, njikwa na-achịkwa, nke na-adịghị ala ala, ule nyocha ụlọ ọgwụ nke multi-center Phase 3, nke na-achọ iji tụnyere ọgwụgwọ nke abụọ nke paclitaxel oral solution RMX3001 na Ịgba ogwu paclitaxel (Taxol) Ịrụ ọrụ na nchekwa na ndị ọrịa nwere ọrịa kansa afọ dị elu.Ọkammụta Li Jin si n'ụlọọgwụ Shanghai Oriental na Prọfesọ Qin Shukui sitere na ụlọ ọgwụ Nanjing Jinling jikọrọ aka mee ọmụmụ a dịka ndị isi nyocha.
Dr. Ruiping Dong, onye isi ọrụ nke Haihe Pharmaceuticals, kwuru, sị: "Nkwenye nke ngwa maka paclitaxel oral solution (RMX3001) bụ ihe ọzọ dị mkpa dị mkpa maka Haihe Pharmaceuticals, na enwere m ekele dị ukwuu nye ndị nyocha ụlọ ọgwụ na ndị ọrịa ndị sonyere na anyị. ikpe.Ọrịa kansa afọ dị elu A ka nwere nnukwu mkpa ụlọ ọgwụ na-enwetaghị ọgwụgwọ, anyị na-atụkwa anya iwetara ndị ọrịa nọ na China na gburugburu ụwa ọgwụgwọ kachasị mma n'ụwa ngwa ngwa o kwere mee.”
Yunnan Hande Biotechnology Co., Ltd nọ na-elekwasị anya na mmepụta nke paclitaxel kemgbe afọ 28.Ọ bụ ụlọ ọrụ izizi nọọrọ onwe ya n'ụwa nke paclitaxel ọgwụ anticancer sitere na osisi nke US FDA, European EDQM, Australian TGA, China CFDA, India, Japan na ụlọ ọrụ nchịkwa mba ndị ọzọ kwadoro.ụlọ ọrụ.Ọ bụrụ na ị chọrọ ịzụPaclitaxel API,biko kpọtụrụ anyị online.
Oge nzipu: Sep-14-2022